Only Lovo delivers automated, functionally closed cell processing through spinning membrane filtration.
See how Lovo brings greater efficiency and consistency to your lab. >
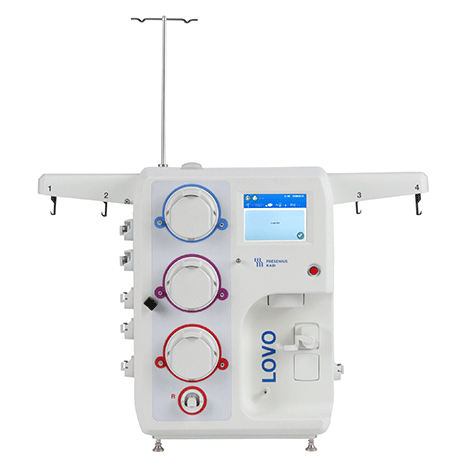
Designed with you in mind.
Hear the people behind Lovo talk about how its technology, design, and performance were driven from the needs of labs like yours.
Each cell therapy manufacturing process is unique. Lovo's flexibility supports multiple applications, at any stage of your operation.
Whether you're working with millions or billions of cells, in 10mL or 22L, Lovo adapts with you to easily scale your process, from Phase 1 through Commercialization.
You don't need to trade cell recovery with washout efficiency. Lovo’s spinning membrane filtration allows you to process a wide range of cell volumes and concentrations quickly, while maximizing recovery and cell viability.
Lovo DXT allows for secure, wired or wireless data transfer. Generate reports, filter procedure records, and export directly to Microsoft Excel or your LIMS.
Lovo seamlessly incorporates into the ScaleReady cell therapy manufacturing platform, enabling you to rapidly generate and package life-saving cell therapies at scale. ScaleReady is currently supporting account management and application support for customers in the United States, Canada, Europe, and Israel.
Learn more about the ScaleReady Platform >

Stay Informed.
Sign up below to receive the latest Lovo news and updates.
See Us In Action.
Come see what Lovo’s all about. We’ll walk you through the features and arrange a demo.
6th Annual TIL Therapies Summit
October 18, 2024
Boston, MA
ESGCT Annual Congress
October 22-25, 2024
La Nuvola, Rome, Italy
Society for Immunotherapy of Cancer (sitc)
November 6-10, 2024
Houston, TX
Document
The LOVO Cell Processing system is for laboratory use only. Unless the user has obtained advance clearance or approval from the appropriate regulatory agency, cells processed on this system are not intended for diagnostic purposes, direct transfusion, or for use in the production of therapeutic products or vaccines for clinical use. For applications requiring regulatory clearance or approval, users may request the required LOVO technical documentation from Fresenius Kabi to support their submissions.
Refer to the LOVO Cell Processing System Operator’s Manual for a complete list of warnings and precautions associated with the use of this device.
References
About Fresenius Kabi USA
Fresenius Kabi is a global health care company that specializes in lifesaving medicines and technologies for infusion, transfusion and clinical nutrition.
Our products are used to help care for critically and chronically ill patients. The people of Fresenius Kabi are driven by a common purpose to put lifesaving medicines and technologies in the hands of people who care for patients, and to find answers to the challenges they face.